The Ultimate Guide to PT-141 Peptide Therapy in Palm Beach & Port St. Lucie: Dosage, Benefits, and Results
Sarah sits in my North Palm Beach office. She’s 52. Successful attorney. Married 28 years. “Dr. Kumar,” she says quietly, “my husband thinks I’m not attracted to him anymore. But it’s not that. I just… don’t feel anything. No desire. No interest. It’s like that part of me died after menopause.”
She’s tried everything her gynecologist suggested: hormone creams, “date nights,” couples therapy. Nothing worked. Her marriage is suffering. Her self-identity is crumbling. “I feel broken,” she whispers. I review her hormone panel. Estrogen, testosterone, thyroid—all optimized. The problem isn’t hormonal. It’s neurological. Her brain’s arousal pathways have gone silent. “Sarah,” I tell her, “you’re not broken. Your melanocortin receptors—the brain circuits responsible for sexual desire—need reactivation.
We’re going to use PT-141, a peptide that works directly on your hypothalamus to restore the neurological signal for arousal. This isn’t about forcing blood flow like Viagra. This is about reigniting desire at the source.” Six weeks later, Sarah returns. She’s smiling. “I feel like myself again. The spontaneous desire I thought was gone forever—it’s back. My husband asked what changed. I told him I finally found a doctor who understood the problem.”
The Problem: The Invisible Barrier to Intimacy
You used to want intimacy. Spontaneously. Naturally. Without effort.
Now? Nothing.
The physical mechanics might work. You can achieve arousal when you try hard enough. But the desire—the actual want—has vanished.
For women, it feels like a chore you’re obligated to perform. You love your partner, but the spark is gone. You feel guilty. Disconnected. Less feminine.
For men, it’s different but equally devastating. You might achieve erections with medication, but the drive isn’t there. The spontaneous morning desire. The mental arousal from attraction. It’s been replaced by performance anxiety and mechanical effort.
This isn’t just about sex. This is about losing part of your identity.
The Neurological Disconnect
Most physicians—and most patients—misunderstand the problem.
They think low libido is:
- Hormonal (low testosterone, low estrogen)
- Vascular (poor blood flow)
- Psychological (stress, depression, relationship issues)
Sometimes, those factors contribute. But often, the problem is neurological.
Sexual desire originates in the hypothalamus—the brain’s command center for appetite, arousal, and motivation. Specifically, the melanocortin system regulates sexual arousal through MC3 and MC4 receptors.
When these receptors aren’t functioning properly:
- The brain doesn’t generate desire signals
- Physical stimulation doesn’t translate into mental arousal
- You can force the mechanics, but genuine want doesn’t emerge
Your brain and body are no longer speaking the same language.
Why Standard Treatments Fail
For Men: Viagra, Cialis, and the Vascular-Only Approach
PDE-5 inhibitors (Viagra, Cialis) work by enhancing blood flow to erectile tissue. They’re effective for mechanical function—if desire already exists.
But if the neurological signal for arousal never fires, enhanced blood flow is useless. It’s like having a car with a full gas tank but a broken ignition switch. The engine won’t start no matter how much fuel you have.
Men in Palm Beach Gardens and Jupiter come to me frustrated: “Viagra works sometimes, but I don’t feel anything. It’s mechanical. The desire isn’t there.”
That’s because Viagra doesn’t activate desire. It only facilitates the mechanics once desire exists.
For Women: Hormones Aren’t Always the Answer
Many women are prescribed testosterone or estrogen creams for low libido. Sometimes this helps, especially in cases of clear hormonal deficiency.
But many women have optimized hormones and still no desire. Their gynecologist says “everything looks normal” and suggests the problem is “psychological” or “just aging.”
This dismissal is medically negligent.
Female Sexual Interest/Arousal Disorder (FSIAD) and Hypoactive Sexual Desire Disorder (HSDD) affect 10-15% of women. These are neurological conditions, not simply hormone deficiencies.
The melanocortin system regulates female sexual desire independently of estrogen and testosterone. When it’s dysfunctional, no amount of hormone cream will restore spontaneous arousal.
The Agitation: The Cost of a Quiet Bedroom
Fading libido is a slow-motion disaster.
It doesn’t happen overnight. It’s gradual. Subtle. You make excuses: “I’m tired.” “Work is stressful.” “Maybe next week.”
Eventually, you stop making excuses because your partner stops asking.
For Men: The Performance Anxiety Spiral
You try Viagra. It works mechanically—sometimes. But the pressure builds.
“Will it work this time?”
That anxiety kills whatever residual desire existed. You start avoiding intimacy because failure feels worse than abstinence.
Your confidence erodes—not just sexually, but in all areas. The competitive drive, the assertiveness, the edge you had in business and relationships—it all dulls.
Your partner wonders if you’re still attracted to them. You wonder if you’re broken. The silence between you grows.
For Women: The Identity Crisis
You’re told desire will return “when you relax” or “when stress decreases” or “after counseling.”
But stress decreases. Counseling happens. Nothing changes. Supplements for treating ED may offer an additional approach for those still looking for solutions.
You start feeling like you’re failing at something fundamental to being a woman. Intimacy becomes an obligation you dread. Guilt compounds.
Your partner feels rejected. You feel inadequate. The emotional distance becomes permanent.
The Relationship Cascade
Lack of physical intimacy destroys more than just sex:
- Emotional connection erodes: Physical intimacy releases oxytocin and dopamine—bonding chemicals
- Resentment builds: One partner feels rejected, the other feels pressured
- Communication breaks down: The topic becomes too painful to discuss
- Affairs become tempting: Not from lack of love, but from desperation for feeling desired again
- Divorce becomes thinkable: What was once unimaginable starts feeling inevitable
You live in Palm Beach or Port St. Lucie to enjoy life fully. Don’t let neurological dysfunction steal your most important relationship.
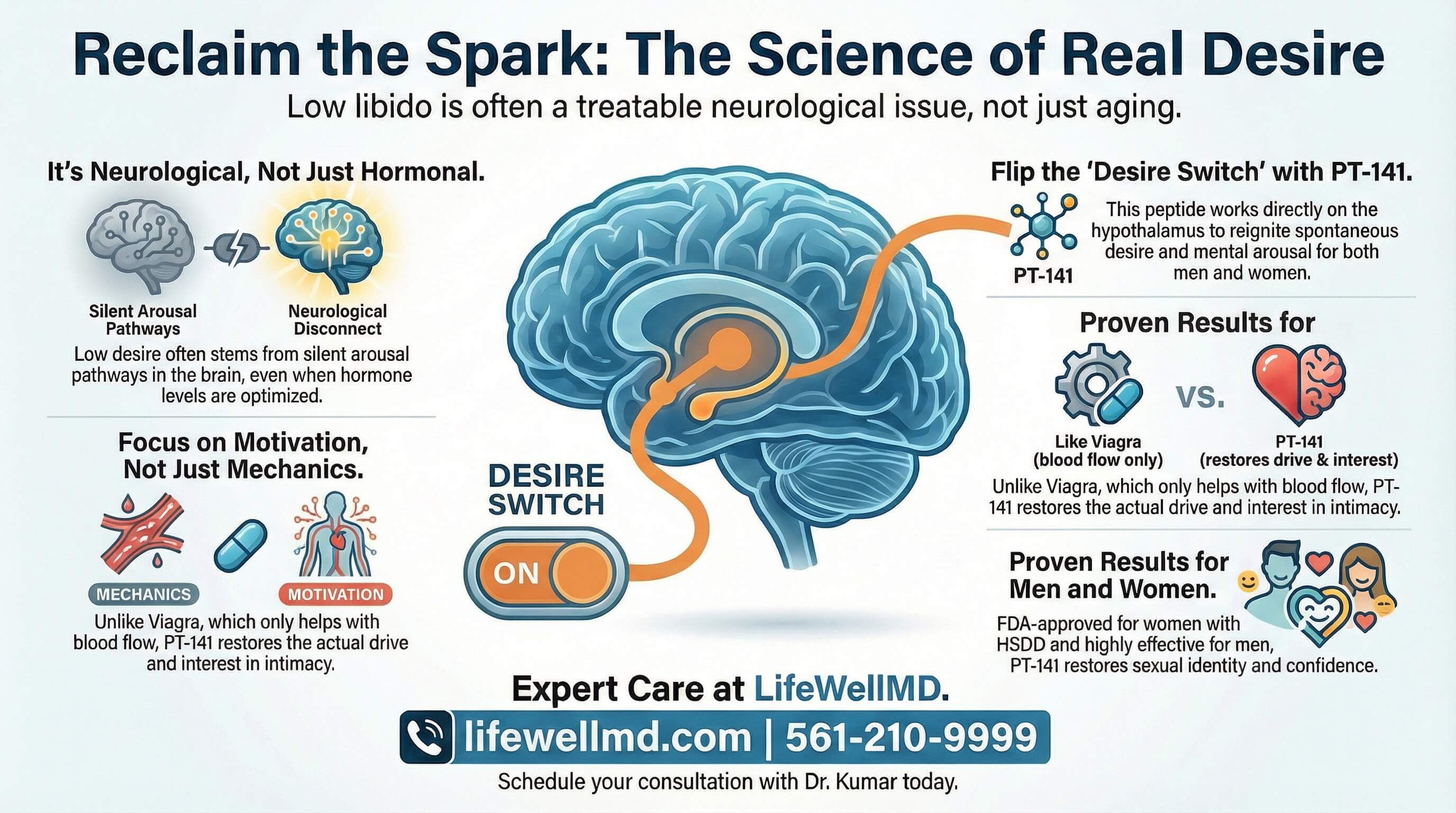
The Solution: PT-141—The “Central” Key to Arousal
PT-141 (Bremelanotide) is not another erectile dysfunction drug.
It’s a melanocortin receptor agonist that works directly on the brain’s arousal centers.
The Mechanism: How PT-141 Actually Works
PT-141 is a synthetic peptide derived from melanotan II. It acts as an agonist at MC3 and MC4 receptors in the hypothalamus—the brain regions responsible for sexual arousal and appetite.
Step 1: Receptor Activation
When PT-141 binds to melanocortin receptors, it triggers a cascade of neurochemical events. For those interested in other hormone-based therapies, such as testosterone cream for women, learn how such treatments can help address specific health concerns and improve overall well-being:
- Activation of neural pathways associated with arousal
- Release of dopamine (pleasure and motivation neurotransmitter)
- Increased nitric oxide production (vasodilation in sexual tissues)
- Enhanced sensitivity to sexual stimuli
Step 2: Central Nervous System Response
Unlike Viagra (which works peripherally on blood vessels), PT-141 works centrally—in the brain itself.
This creates:
- Spontaneous desire: Not just ability to respond, but actual wanting
- Mental arousal: Thoughts and fantasies return
- Emotional engagement: Sex feels connected to emotion, not mechanical
- Enhanced sensation: Increased sensitivity to touch and stimulation
Step 3: Peripheral Effects
While PT-141’s primary action is central, it also produces peripheral effects:
- Increased blood flow to genital tissues
- Enhanced lubrication in women
- Improved erectile quality in men
- Reduced refractory period (time between orgasms)
This is whole-system arousal activation, not isolated mechanical function.
PT-141 vs. Traditional ED Medications
For information on the impact of semaglutide on libido and sexual health, see this guide.
Comparison Table:
| Kisspeptin 10 Dosage: The Ultimate Guide Feature | PT-141 (Bremelanotide) | Viagra/Cialis (PDE-5 Inhibitors) |
|---|---|---|
| Mechanism | Melanocortin receptor agonist (brain-based) | Phosphodiesterase-5 inhibitor (vascular) |
| Primary Action | Increases sexual desire and arousal | Enhances erectile blood flow |
| Works For | Men and women with desire disorders | Men with erectile dysfunction only |
| Onset | 30 minutes to 4 hours | 30-60 minutes |
| Duration | 24-72 hours of enhanced responsiveness | 4-36 hours (depending on drug). For those interested in natural alternatives, consider exploring the best gummies for ED for improved performance. |
| Requires Arousal | No—creates spontaneous desire | Yes—only works if desire exists |
| Hormonal | Non-hormonal | Non-hormonal |
| FDA Status | FDA-approved for HSDD in women | FDA-approved for ED in men |
The Critical Difference:
Viagra asks: “Can you get an erection if you want one?”
PT-141 asks: “Do you actually want one?”
For thousands of people of all sexes, the answer to the second question is no—and that’s the real problem.
Clinical Benefits & Results: What PT-141 Delivers
For Women: Restoration of Desire
Primary Benefit: Treatment of HSDD (Hypoactive Sexual Desire Disorder)
PT-141 is FDA-approved specifically for premenopausal women with HSDD. Clinical trials demonstrate:
- 50-60% of women report meaningful improvement in sexual desire
- Increased frequency of satisfying sexual encounters
- Improved emotional connection during intimacy
- Reduced distress about low libido
Secondary Benefits:
- Enhanced arousal and lubrication
- Increased sensitivity to stimulation
- More frequent orgasms
- Restoration of sexual identity and confidence
Women in North Palm Beach and Stuart report feeling “like themselves again” after PT-141 therapy—the spontaneous desire they thought menopause had permanently stolen returns.
For Men: Desire + Function
Primary Benefits:
Restored Libido: The mental component of sexual desire returns. Spontaneous arousal. Sexual thoughts. The drive that existed in your 30s.
Enhanced Erectile Quality: Not just blood flow (like Viagra), but neurologically-driven erections that feel natural and responsive.
Reduced Refractory Period: Faster recovery between sexual encounters. Many men report ability for multiple encounters in a session—something they haven’t experienced in years.
Improved Orgasm Intensity: Enhanced sensation and more powerful orgasms due to increased central nervous system activation.
Psychological Benefits: See real-life examples
- Elimination of performance anxiety (desire is genuine, not forced)
- Restored sexual confidence
- Improved relationship satisfaction
- Enhanced overall motivation and drive
Combined with Testosterone: Synergistic Effects
Many men in the “Gray Zone” (testosterone 350-550 ng/dL) benefit from combining testosterone optimization with PT-141.
Testosterone provides:
- Baseline hormonal support for libido
- Muscle mass and energy
- Metabolic health
PT-141 adds:
- Neurological arousal activation
- Spontaneous desire
- Enhanced mental engagement
The combination produces superior results to either therapy alone.
Dr. Kumar explains how PT-141 activates the brain’s desire pathways, why it works for folks of all genders when Viagra doesn’t, and the precise protocol for restoring spontaneous arousal
The LifeWellMD Protocol: Expert Dosage & Administration
As a Board-Certified Radiation Oncologist with over 30 years experience in precision medicine, I approach PT-141 therapy with clinical rigor—not the “shotgun approach” of online peptide mills.
Administration & Dosing
PT-141 Protocol Specifications:
| Parameter | Specification |
|---|---|
| Administration Route | Subcutaneous injection (abdomen or thigh) |
| Injection Technique | Self-administered with insulin syringe (same as diabetes injections) |
| Standard Dose Range | 1.0 mg to 2.0 mg per dose |
| Personalized Dosing | Customized based on body weight, sensitivity, and response |
| Onset of Action | 30 minutes to 4 hours (average 1-2 hours) |
| Peak Effect | 2-3 hours post-injection |
| Duration of Effect | 24-72 hours of enhanced sexual responsiveness |
| Frequency | As needed, typically 2-3 times weekly maximum |
| Training: For those interested in natural wellness approaches, explore the potential benefits of Kisspeptin Supplement: A Natural Libido Boost. | Hands-on injection technique instruction at initial visit |
The Patient Journey at LifeWellMD
Step 1: Comprehensive Evaluation (Week 1)
Before prescribing PT-141, I evaluate:
- Complete sexual health history: Understanding the nature and duration of symptoms
- Hormone panel: Total testosterone, free testosterone, estradiol, DHEA, thyroid
- Cardiovascular screening: Blood pressure, heart rate, cardiovascular history
- Medication review: Checking for interactions or contraindications
- Relationship assessment: Understanding partner dynamics and goals
PT-141 is not appropriate for everyone. Men with uncontrolled hypertension or cardiovascular disease require careful evaluation.
Step 2: Initial Dosing & Training (Week 1-2)
- Prescription of pharmaceutical-grade PT-141 from accredited compounding pharmacy
- Hands-on injection technique training (subcutaneous injection is simple—most patients master it in one session)
- Clear instructions on timing (inject 45-90 minutes before anticipated intimacy)
- Side effect management protocols
Step 3: Titration & Optimization (Weeks 2-8)
- Initial dose: Typically 1.0 mg for women, 1.5 mg for men
- Response assessment at 2-week follow-up
- Dose adjustment based on efficacy and side effects
- Most patients find optimal dose within 3-4 trials
Step 4: Maintenance & Integration (Ongoing)
- PT-141 used as-needed (not daily medication)
- Integration with other therapies when appropriate (testosterone, peptides, PRP) — an important strategy for addressing erectile dysfunction in your 20s
- Quarterly follow-ups to assess continued efficacy and safety
- Ongoing support and protocol adjustments
Side Effects & Management
PT-141 is generally well-tolerated. Common side effects include:
Mild/Transient:
- Facial flushing (20-30% of patients)
- Mild nausea (15-20%, usually first 1-2 doses)
- Headache (10-15%)
- Temporary increase in blood pressure (monitored carefully)
Management Strategies:
- Starting with lower doses and titrating up
- Taking with light food to reduce nausea
- Hydration before and after injection
- Anti-nausea medication if needed for first few doses
Serious side effects are rare when properly screened and monitored.
Contraindications
PT-141 is not appropriate for:
- Uncontrolled hypertension (BP >160/100)
- Recent cardiovascular events (heart attack, stroke)
- Pregnancy or breastfeeding
- Active cardiovascular disease requiring nitrate medications
This is why physician oversight matters. Online peptide sources cannot provide proper medical screening.
Why LifeWellMD Is the Authority in South Florida Peptide Therapy
Board-Certified Physician, Not a Peptide Mill
Most “peptide clinics” in South Florida are:
- Staffed by physician assistants or nurse practitioners
- Following generic protocols regardless of individual needs
- Sourcing peptides from unverified overseas suppliers
- Providing zero medical oversight or safety monitoring
I am Dr. Ramesh Kumar, MD—Board-Certified Radiation Oncologist with Harvard Medical School training and 30+ years treating complex medical conditions in over 10,000 patients.
I understand cellular biology. I understand receptor pharmacology. I understand the cardiovascular risks that require monitoring.
When you receive PT-141 from LifeWellMD, you’re receiving physician-led precision medicine—not mail-order peptides with no medical support.
Pharmaceutical-Grade Peptides Only
We source PT-141 exclusively from:
- FDA-registered 503B compounding pharmacies
- Facilities with current Good Manufacturing Practice (cGMP) certification
- Third-party tested for purity and potency
Generic overseas peptide powders are unregulated, often contaminated, and potentially dangerous.
The price difference isn’t worth the risk.
Integrated Approach
PT-141 works best when integrated with comprehensive sexual health optimization:
For Men:
- Testosterone optimization (if in Gray Zone)
- Cardiovascular health assessment
- P-Shot (PRP) for vascular enhancement when needed
- Trimix for severe cases requiring additional support
For Women:
- Hormone optimization (estrogen, progesterone, testosterone)
- Vaginal rejuvenation (O-Shot PRP therapy)
- Pelvic floor assessment and treatment
- Relationship counseling when appropriate
This is comprehensive sexual health restoration, not just peptide prescriptions.
Local, Accessible Care
You’re not dealing with an online telehealth service that disappears when you need support.
Two convenient locations:
- North Palm Beach: Serving Palm Beach Gardens, Jupiter, West Palm Beach, Juno Beach
- Port St. Lucie: Serving Treasure Coast, Stuart, Fort Pierce, Vero Beach, Palm City
You see Dr. Kumar personally at every visit. Your sexual health is my personal responsibility.
Frequently Asked Questions: PT-141 Peptide Therapy
How quickly does PT-141 work?
PT-141 typically produces effects within 30 minutes to 4 hours after injection, with most patients experiencing peak effects at 1-3 hours. Unlike daily medications, PT-141 is used on-demand before anticipated intimacy. The enhanced sexual responsiveness can persist for 24-72 hours after a single dose, meaning spontaneous desire may emerge even the day after injection.
Is PT-141 safe for women after menopause?
Yes. PT-141 is FDA-approved for premenopausal women with HSDD, but clinical experience shows significant benefits for postmenopausal women as well. Since PT-141 works through melanocortin receptors (not hormonal pathways), it remains effective regardless of menopausal status. At LifeWellMD, we often combine PT-141 with bioidentical hormone therapy for optimal results in postmenopausal women.
Can PT-141 replace Viagra or Cialis?
For some men, yes. PT-141 works through a completely different mechanism—activating brain-based desire pathways rather than just enhancing blood flow. Men who respond well to PT-141 often discontinue PDE-5 inhibitors because the neurological arousal is more natural and satisfying. However, some men benefit from combining both—PT-141 for desire and Viagra for additional vascular support. Dr. Kumar customizes protocols based on individual response.
What are the side effects of PT-141?
Most common side effects are mild and transient: facial flushing (20-30%), mild nausea (15-20%, usually first 1-2 doses), headache (10-15%), and temporary blood pressure elevation. These typically resolve within a few hours and diminish with continued use. Starting with lower doses and proper hydration minimizes side effects. Serious side effects are rare when patients are properly screened for cardiovascular contraindications.
How much does PT-141 therapy cost at LifeWellMD?
Initial consultation including comprehensive evaluation and training ranges from $400-$600. PT-141 peptide cost is $150-$250 per vial (containing multiple doses, typically 4-8 depending on your prescribed dose). Most patients use 2-3 doses weekly, making monthly cost approximately $200-$400. This is an out-of-pocket investment as insurance rarely covers peptide therapy, but HSA/FSA funds can be used.
Can I buy PT-141 online without a doctor?
Legally, no. PT-141 is a prescription medication. Websites selling “research peptides” without prescription are operating illegally and providing unregulated, potentially contaminated products. More importantly, PT-141 requires medical screening—particularly cardiovascular evaluation—to ensure safety. Men with uncontrolled hypertension or recent cardiovascular events should not use PT-141. Only a physician can determine if you’re an appropriate candidate.
Does PT-141 work ?
Yes—this is one of PT-141’s unique advantages. It’s FDA-approved for women with HSDD and widely used off-label for men with desire disorders. The melanocortin receptors PT-141 activates exist in both male and female brains. Women experience restored desire, increased arousal, and enhanced sensitivity. Men experience increased libido, improved erectile quality, and reduced refractory period. It’s one of the few sexual health treatments effective for both sexes.
How long does it take to see results from PT-141?
Effects begin within 30 minutes to 4 hours of injection and can last 24-72 hours. However, full benefits often emerge over 4-8 weeks as patients find their optimal dose and timing. The first dose may produce noticeable desire increase but require titration for best results. Most patients report significant improvement by their third or fourth dose once we’ve customized their protocol.
Can I use PT-141 if I have cardiovascular disease?
This requires careful physician evaluation. PT-141 can cause temporary blood pressure elevation (typically 10-15 mmHg), which is usually well-tolerated by healthy individuals but potentially problematic for those with uncontrolled hypertension or recent cardiovascular events. As a Board-Certified physician, Dr. Kumar evaluates cardiovascular risk, reviews medications for interactions, and determines if PT-141 is safe for your specific situation. Some patients require cardiovascular optimization before starting PT-141.
How is PT-141 different from testosterone therapy?
PT-141 and testosterone work through completely different mechanisms. Testosterone provides hormonal foundation for libido, energy, muscle mass, and metabolic health—but doesn’t directly activate brain arousal pathways. PT-141 works on melanocortin receptors to create neurological desire signals. Many men in the Gray Zone benefit from combining both: testosterone for baseline hormonal support and PT-141 for arousal activation. Women can use PT-141 regardless of hormone status.
Take Action: Reclaim Intimacy and Vitality
You’ve lived too long accepting diminished desire as “normal”—discover how personalized, holistic healthcare solutions can help you reclaim your well-being.
It’s not normal. It’s neurological dysfunction—and it’s treatable.
Every month you delay is another month of:
- Emotional distance in your most important relationship
- Confidence erosion affecting all areas of life
- Opportunities for intimacy missed and forgotten
- Your partner wondering if you’re still attracted to them
- Guilt about not “being enough”
Stop settling. Start living fully.
Why Wait When the Solution Exists?
You live in Palm Beach County or the Treasure Coast to enjoy life at its fullest. Golf at PGA Village. Boating the Intracoastal. Building successful careers. Maintaining vibrant relationships.
Don’t let neurological arousal dysfunction steal your vitality.
PT-141 therapy can restore what you thought was permanently lost:
- Spontaneous sexual desire
- Mental arousal and fantasies
- Emotional connection during intimacy
- Sexual confidence and identity
- Relationship satisfaction and closeness
This isn’t about “performance.” This is about feeling like yourself again.
Your Next Step
Call LifeWellMD today: 561-210-9999 | Learn more about P-Shot cost and outcomes
Schedule your confidential sexual health consultation with Dr. Kumar.
Two discreet, professional locations:
- North Palm Beach Clinic (serving Palm Beach Gardens, Jupiter, West Palm Beach)
- Port St. Lucie Clinic (serving Treasure Coast, Stuart, Fort Pierce, Vero Beach)
Visit LifeWellMD.com to learn more about our sexual health optimization protocols.
What You’ll Receive:
- Comprehensive 60-minute evaluation with Dr. Kumar reviewing your sexual health history, relationship dynamics, and treatment goals
- Complete hormone and cardiovascular screening to ensure PT-141 safety and identify complementary therapies
- Customized PT-141 protocol with precise dosing based on your physiology and response
- Hands-on injection training (simple subcutaneous technique you’ll master in minutes)
- Pharmaceutical-grade peptides from FDA-registered 503B compounding pharmacies
- Ongoing physician support with dose adjustments and protocol optimization
This is physician-led sexual health restoration—not mail-order peptides with no medical oversight.
Don’t spend another year in a quiet bedroom, wondering if the spark is gone forever.
561-210-9999
Because intimacy matters. Desire matters. Your relationship deserves better than resignation.
Dr. Ramesh Kumar is a Board-Certified Radiation Oncologist with Harvard Medical School training and over 30 years experience treating complex medical conditions in more than 10,000 patients. After founding four cancer centers, Dr. Kumar now focuses on integrative and regenerative medicine at LifeWellMD in North Palm Beach and Port St. Lucie, Florida.
His expertise in cellular biology, receptor pharmacology, and precision medicine allows him to provide comprehensive sexual health optimization including PT-141 peptide therapy for men and women struggling with desire disorders. Dr. Kumar specializes in restoring neurological arousal pathways that conventional medicine dismisses as “normal aging.”
Please check out his 120 five star reviews on Healthgrades and his 136 five star reviews at WebMD.


